Blog
The Antibody Developability Crisis: Why 40% of Candidates Fail
Dec 15, 2025
You've identified a high-affinity antibody. It binds your target with nanomolar affinity. The selectivity looks perfect. In cell assays, it shows the exact mechanism you want. Then you try to formulate it at 100 mg/mL for subcutaneous injection, and it aggregates into an opaque gel within 24 hours.
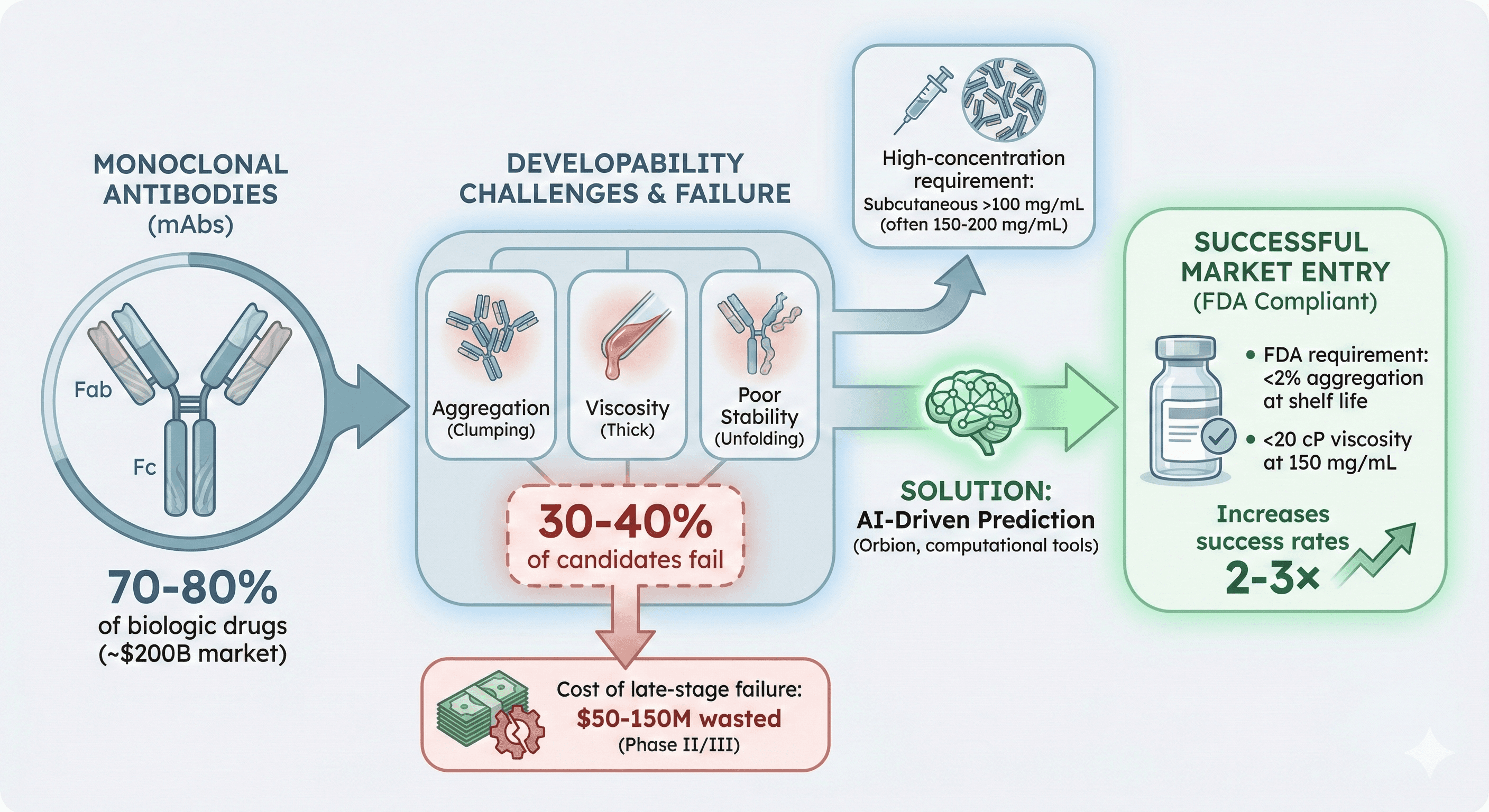
Welcome to the developability problem—the graveyard where 30-40% of antibody drug candidates die after millions of dollars in investment. Here's why it happens, and what makes an antibody "developable."
Key Takeaways
70-80% of biologic drugs are monoclonal antibodies (~$200B market)
30-40% of antibody candidates fail due to developability issues (aggregation, viscosity, poor stability)
Cost of late-stage failure: $50-150M wasted if developability issues found in Phase II/III
High-concentration requirement: Subcutaneous formulations need >100 mg/mL (often 150-200 mg/mL)
Industry standard: <2% aggregation at shelf life, <20 cP viscosity at 150 mg/mL
The problem: Traditional discovery optimizes for affinity only; developability is an afterthought
The Developability Crisis in Numbers
Market Size vs Failure Rate
The antibody market:
Monoclonal antibodies: $200B+ global market
Top-selling drugs: Humira ($21B/year), Keytruda ($17B/year), Opdivo ($7B/year)
Pipeline: 1,000+ antibodies in clinical trials
70-80% of approved biologics are antibodies
The failure rate:
30-40% of antibody candidates fail due to developability issues
These failures happen late (Phase II/III or even during manufacturing scale-up)
Average cost per failure: $50-150M
Time lost: 3-5 years
What kills antibodies:
Aggregation (40% of failures): Forms oligomers, loses activity, becomes immunogenic
High viscosity (25%): Cannot inject through small-gauge needle (patient compliance)
Poor stability (20%): Degrades during storage, short shelf life
Immunogenicity (10%): Anti-drug antibodies (ADAs) neutralize therapeutic
Low expression (5%): Cannot manufacture at commercial scale (>3 g/L in CHO)
The Economic Reality
Traditional development timeline:
Discovery: 1-2 years
Lead optimization: 1-2 years
IND-enabling studies: 1 year
Phase I: 1-2 years
Phase II: 2-3 years
Phase III: 2-4 years
Total: 8-14 years, $1-2B per approved drug
When developability failure is discovered:
Early (preclinical): Waste $10-30M, restart with new candidate
Phase I: Waste $40-80M
Phase II: Waste $100-200M
Phase III or manufacturing: Waste $300-500M, program may be terminated
The brutal math:
If you discover aggregation issues in Phase II: $150M gone
If viscosity problems appear during scale-up: $300M gone
Success rate drops from 60% → 20% if developability isn't addressed early
What Is Developability?
Developability is the set of biophysical properties that determine whether an antibody can be manufactured, formulated, stored, and administered as a drug.
The Perfect Antibody Candidate
Must have:
High affinity (Kd < 1 nM)
High specificity (no off-target binding)
Desired mechanism (agonist, antagonist, ADCC, CDC)
AND developability:
Soluble at >150 mg/mL (subcutaneous injection requires low volume)
<2% aggregation at 6-24 months shelf life (FDA requirement)
Viscosity <20 cP at 150 mg/mL (injectable through 27-gauge needle)
Stable at 2-8°C (refrigerator storage, patient-friendly)
Tm (melting temperature) >60°C for all domains (thermal stability)
Low immunogenicity risk (no anti-drug antibodies)
Expression titer >3 g/L in CHO cells (commercially viable manufacturing)
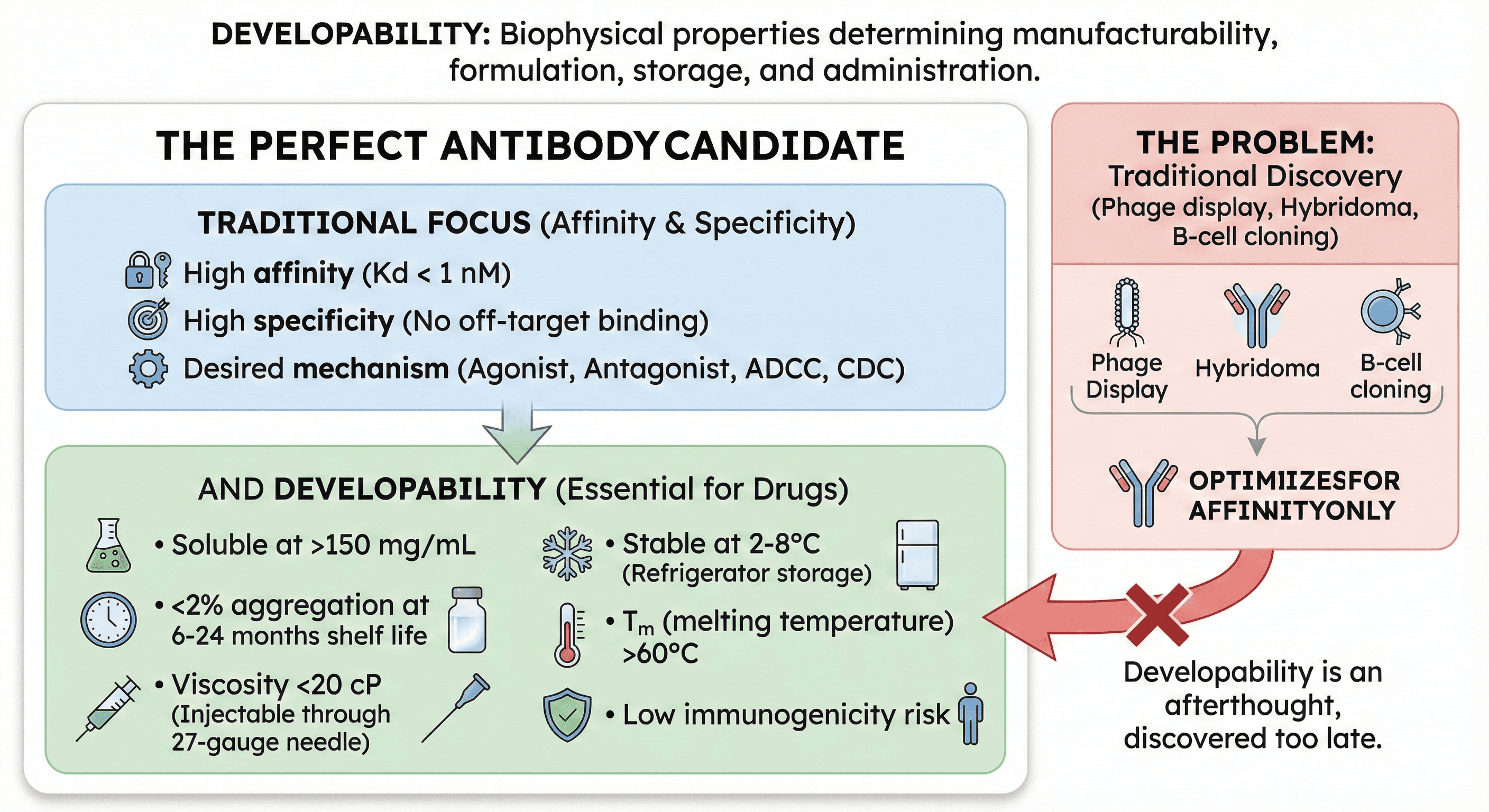
The Fundamental Problem
Traditional antibody discovery (phage display, hybridoma, B-cell cloning):
Optimizes for: Affinity
Screens for: Binding to target antigen
Selection pressure: Highest Kd wins
What's missing:
No selection for solubility
No screening for viscosity
No filtering for stability
No assessment of manufacturability
Result: You get high-affinity antibodies that often have terrible biophysical properties.
The Five Critical Quality Attributes (CQAs)
The FDA evaluates antibodies on these developability criteria. Fail any one, and your drug candidate is in jeopardy.
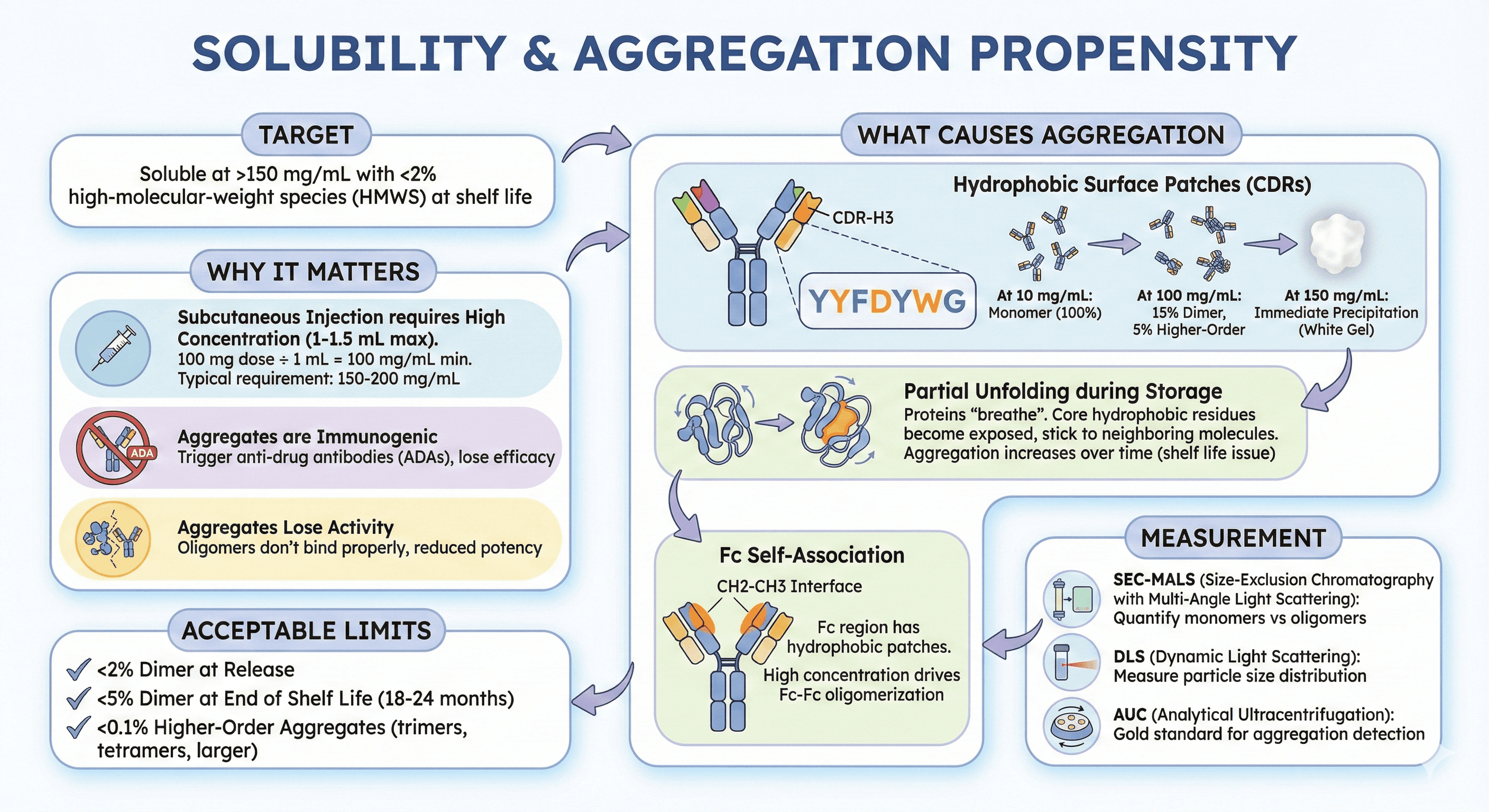
1. Solubility & Aggregation Propensity
Target: Soluble at >150 mg/mL with <2% high-molecular-weight species (HMWS) at shelf life
Why it matters:
Subcutaneous injection requires high concentration (1-1.5 mL max injection volume)
100 mg dose ÷ 1 mL volume = need 100 mg/mL minimum
Typical requirement: 150-200 mg/mL (includes overfill, stability margin)
Aggregates are immunogenic: Trigger anti-drug antibodies (ADAs), lose efficacy
Aggregates lose activity: Oligomers don't bind properly, reduced potency
What causes aggregation:
Hydrophobic surface patches:
Antibody CDRs (complementarity-determining regions) contain the binding site
To bind hydrophobic pockets on antigens, CDRs often have hydrophobic residues (Tyr, Phe, Trp, Leu, Ile)
These hydrophobic CDRs can stick to each other at high concentration
Result: Reversible self-association → irreversible aggregation
Example:
CDR-H3 with sequence YYFDYWG (3 Tyr, 1 Phe, 1 Trp)
At 10 mg/mL: Monomer (100%)
At 100 mg/mL: 15% dimer, 5% higher-order aggregates
At 150 mg/mL: Immediate precipitation (white gel)
Partial unfolding during storage:
Proteins "breathe" (transiently unfold and refold)
During unfolding, core hydrophobic residues become exposed
Exposed regions can stick to neighboring molecules
Result: Aggregation increases over time (shelf life issue)
Fc self-association:
The Fc (constant region) can also aggregate
CH2-CH3 interface has hydrophobic patches
At high concentration, Fc-Fc interactions drive oligomerization
Measurement:
SEC-MALS (size-exclusion chromatography with multi-angle light scattering): Quantify monomers vs oligomers
DLS (dynamic light scattering): Measure particle size distribution
AUC (analytical ultracentrifugation): Gold standard for aggregation detection
Acceptable limits:
<2% dimer at release
<5% dimer at end of shelf life (18-24 months)
<0.1% higher-order aggregates (trimers, tetramers, larger)
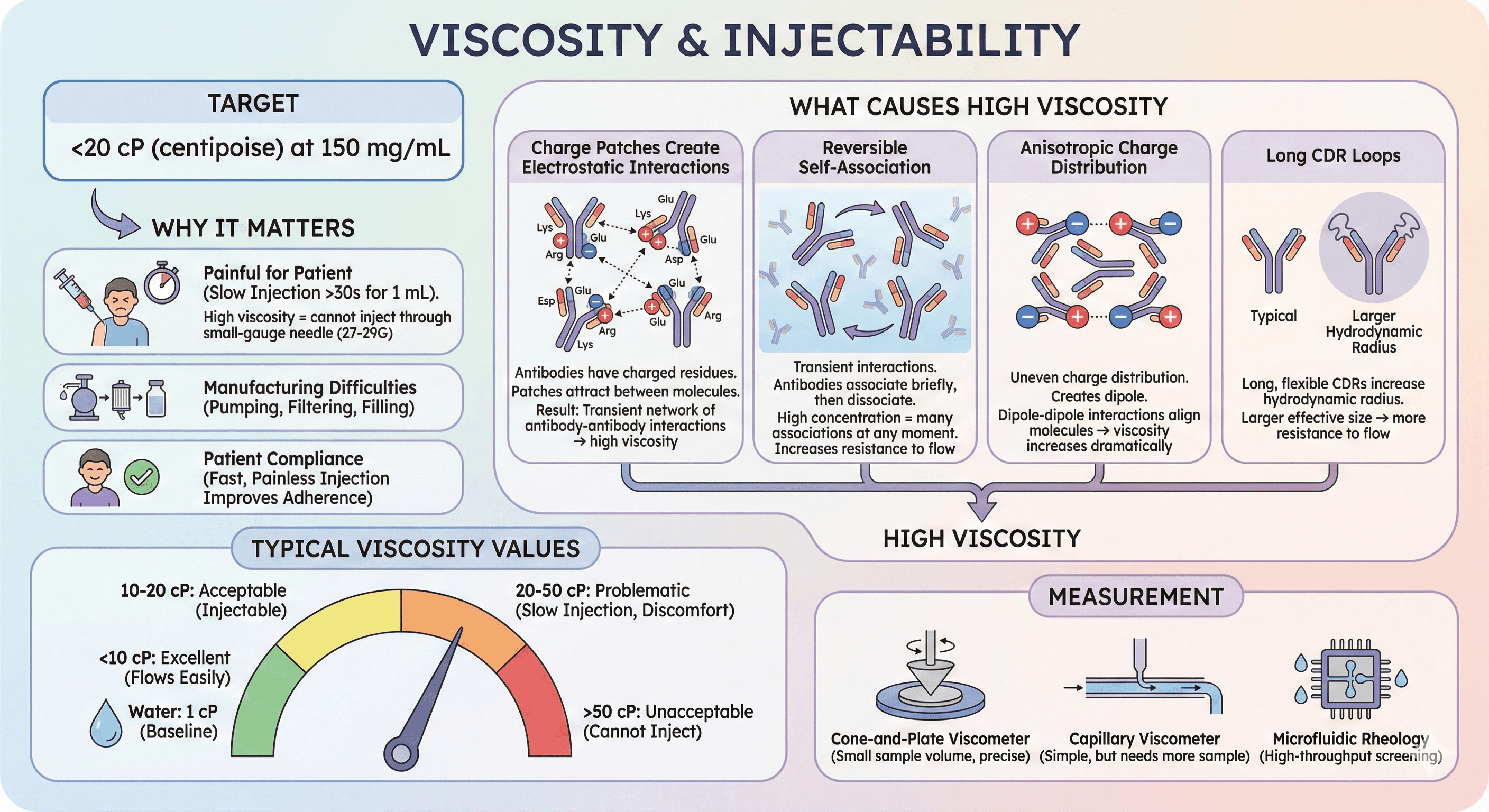
2. Viscosity
Target: <20 cP (centipoise) at 150 mg/mL
Why it matters:
High viscosity = cannot inject through small-gauge needle (27-29G)
Slow injection = painful for patient (>30 seconds for 1 mL)
Manufacturing: Difficult to pump, filter, fill into vials/syringes
Patient compliance: Fast, painless injection improves adherence to treatment
What causes high viscosity:
Charge patches create electrostatic interactions:
Antibodies have charged residues (Lys, Arg = positive; Glu, Asp = negative)
If positive charges cluster on one region (patch), negative charges cluster elsewhere
These patches attract between molecules at high concentration
Result: Transient network of antibody-antibody interactions → high viscosity
Reversible self-association:
Unlike aggregation (irreversible), viscosity involves transient interactions
Antibodies associate briefly, then dissociate
At high concentration, many molecules are associated at any given moment
This increases resistance to flow (viscosity)
Anisotropic charge distribution:
"Anisotropic" = uneven charge distribution
If one side of antibody is positive, other side negative → creates dipole
Dipole-dipole interactions align molecules → viscosity increases dramatically
Long CDR loops:
Long, flexible CDRs increase hydrodynamic radius
Larger effective size → more resistance to flow
Typical viscosity values:
Water: 1 cP (baseline)
<10 cP: Excellent (flows easily)
10-20 cP: Acceptable (injectable)
20-50 cP: Problematic (slow injection, patient discomfort)
50 cP: Unacceptable (cannot inject through standard needles)
Measurement:
Cone-and-plate viscometer (small sample volume, precise)
Capillary viscometer (simple, but needs more sample)
Microfluidic rheology (high-throughput screening)
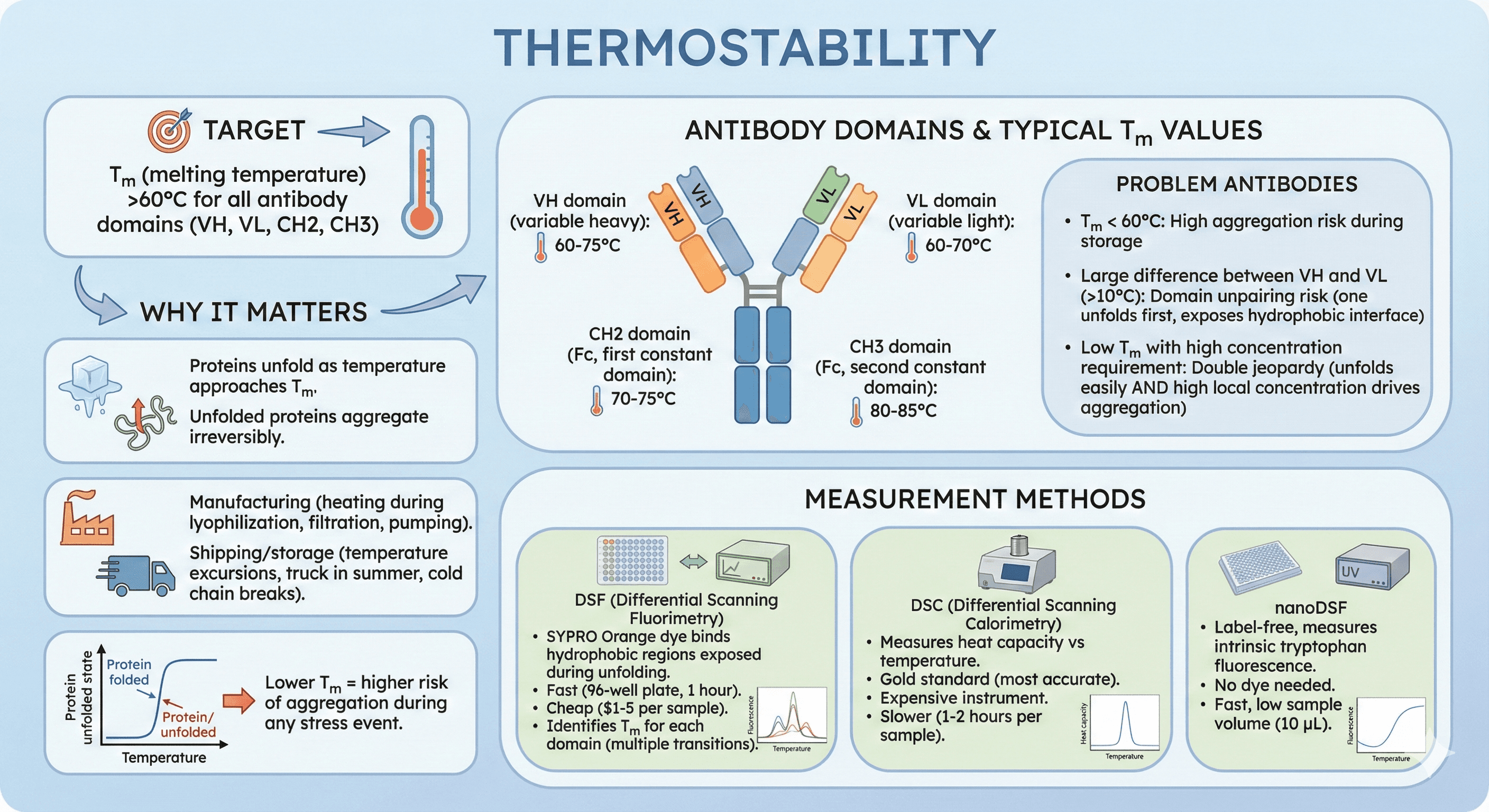
3. Thermal Stability (Tm)
Target: Tm (melting temperature) >60°C for all antibody domains (VH, VL, CH2, CH3)
Why it matters:
Proteins unfold as temperature approaches Tm
Unfolded proteins aggregate irreversibly
During manufacturing: Transient heating can occur (lyophilization, filtration, pumping)
Shipping/storage: Temperature excursions (truck in summer, cold chain breaks)
Lower Tm = higher risk of aggregation during any stress event
Antibody domains and their typical Tm values:
VH domain (variable heavy): 60-75°C
VL domain (variable light): 60-70°C
CH2 domain (Fc, first constant domain): 70-75°C
CH3 domain (Fc, second constant domain): 80-85°C
Problem antibodies:
Tm < 60°C: High aggregation risk during storage
Large difference between VH and VL (>10°C): Domain unpairing risk (one unfolds first, exposes hydrophobic interface)
Low Tm with high concentration requirement: Double jeopardy (unfolds easily AND high local concentration drives aggregation)
Measurement methods:
DSF (Differential Scanning Fluorimetry): SYPRO Orange dye binds hydrophobic regions exposed during unfolding
Fast (96-well plate, 1 hour)
Cheap ($1-5 per sample)
Identifies Tm for each domain (multiple transitions)
DSC (Differential Scanning Calorimetry): Measures heat capacity vs temperature
Gold standard (most accurate)
Expensive instrument
Slower (1-2 hours per sample)
nanoDSF: Label-free, measures intrinsic tryptophan fluorescence
No dye needed
Fast, low sample volume (10 μL)
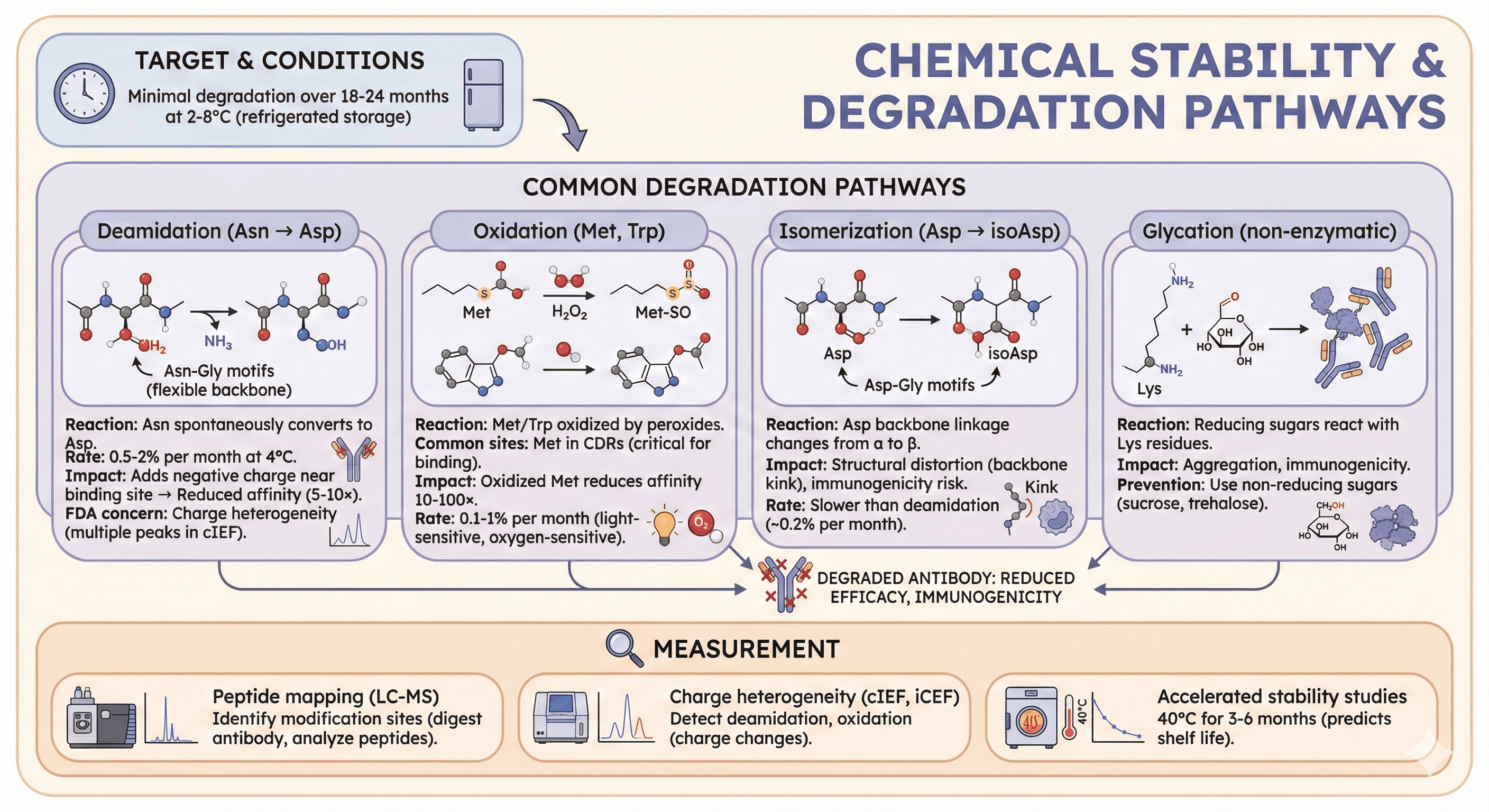
4. Chemical Stability
Target: Minimal degradation over 18-24 months at 2-8°C (refrigerated storage)
Common degradation pathways:
Deamidation (Asn → Asp):
Reaction: Asparagine (Asn) spontaneously converts to Aspartate (Asp)
Susceptible sequences: Asn-Gly motifs (Gly has no side chain, allows backbone flexibility)
Rate: 0.5-2% per month at 4°C (pH-dependent)
Impact: Adds negative charge near binding site → reduced affinity (5-10×)
FDA concern: Charge heterogeneity (multiple peaks in cIEF, charge-based separation)
Oxidation (Met, Trp):
Reaction: Methionine (Met) or Tryptophan (Trp) oxidized by peroxides (H₂O₂, lipid peroxides)
Common sites: Met in CDRs (critical for binding)
Impact: Oxidized Met reduces affinity 10-100× (sulfur becomes sulfoxide or sulfone)
Rate: 0.1-1% per month (light-sensitive, oxygen-sensitive)
Isomerization (Asp → isoAsp):
Reaction: Aspartate (Asp) backbone linkage changes from α to β (isoAsp)
Susceptible sequences: Asp-Gly motifs
Impact: Structural distortion (backbone kink), immunogenicity risk
Rate: Slower than deamidation (~0.2% per month)
Glycation (non-enzymatic):
Reaction: Reducing sugars (glucose, fructose) react with Lys residues
Impact: Aggregation, immunogenicity (glycated proteins recognized as foreign)
Prevention: Use non-reducing sugars (sucrose, trehalose in formulation)
Measurement:
Peptide mapping (LC-MS): Identify modification sites (digest antibody, analyze peptides)
Charge heterogeneity (cIEF, iCEF): Detect deamidation, oxidation (charge changes)
Accelerated stability studies: 40°C for 3-6 months (predicts shelf life)
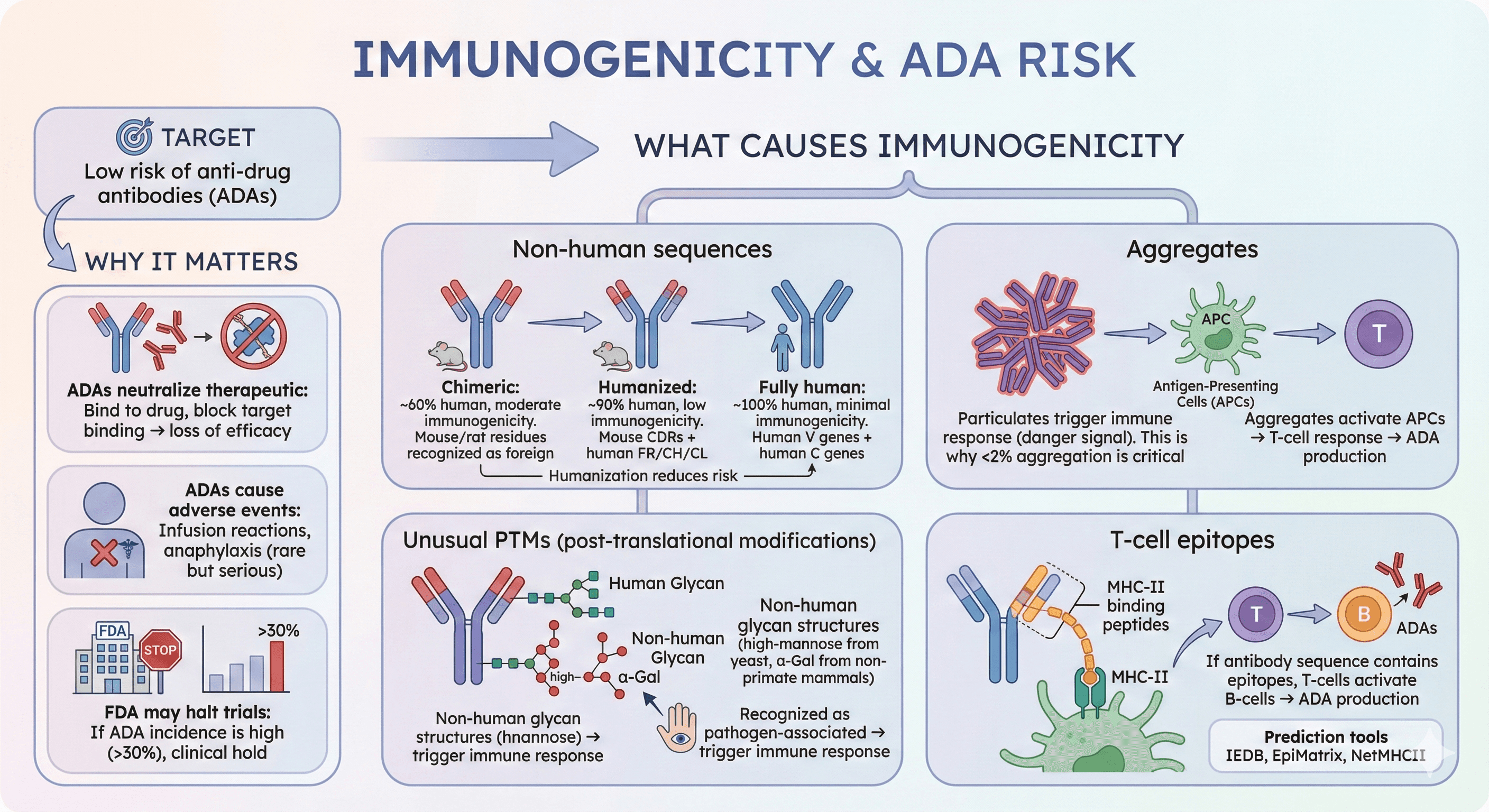
5. Immunogenicity Risk
Target: Low risk of anti-drug antibodies (ADAs)
Why it matters:
ADAs neutralize therapeutic: Bind to drug, block binding to target → loss of efficacy
ADAs cause adverse events: Infusion reactions, anaphylaxis (rare but serious)
FDA may halt trials: If ADA incidence is high (>30%), clinical hold
What causes immunogenicity:
Non-human sequences:
Mouse/rat residues in CDRs or frameworks: Immune system recognizes as foreign
Humanization reduces risk:
Chimeric antibodies (mouse VH/VL + human CH/CL): ~60% human, moderate immunogenicity
Humanized antibodies (mouse CDRs + human FR/CH/CL): ~90% human, low immunogenicity
Fully human antibodies (human V genes + human C genes): ~100% human, minimal immunogenicity
Aggregates:
Particulates trigger immune response (danger signal)
Aggregates activate antigen-presenting cells (APCs) → T-cell response → ADA production
This is why <2% aggregation is critical (FDA requirement)
Unusual PTMs (post-translational modifications):
Non-human glycan structures (high-mannose from yeast, α-Gal from non-primate mammals)
These glycans trigger immune response (recognized as pathogen-associated)
T-cell epitopes:
MHC-II binding peptides (9-15 amino acids)
If antibody sequence contains epitopes, T-cells activate B-cells → ADA production
Prediction tools: IEDB, EpiMatrix, NetMHCII
The Antibody Structure: Where Problems Hide
IgG Architecture Recap
A typical IgG1 antibody (~150 kDa):
2 Heavy chains (50 kDa each): VH-CH1-Hinge-CH2-CH3
2 Light chains (25 kDa each): VL-CL
12 intrachain disulfide bonds (within domains)
4 interchain disulfide bonds (linking chains)
1 N-glycosylation site per heavy chain (Asn297 in CH2 domain)
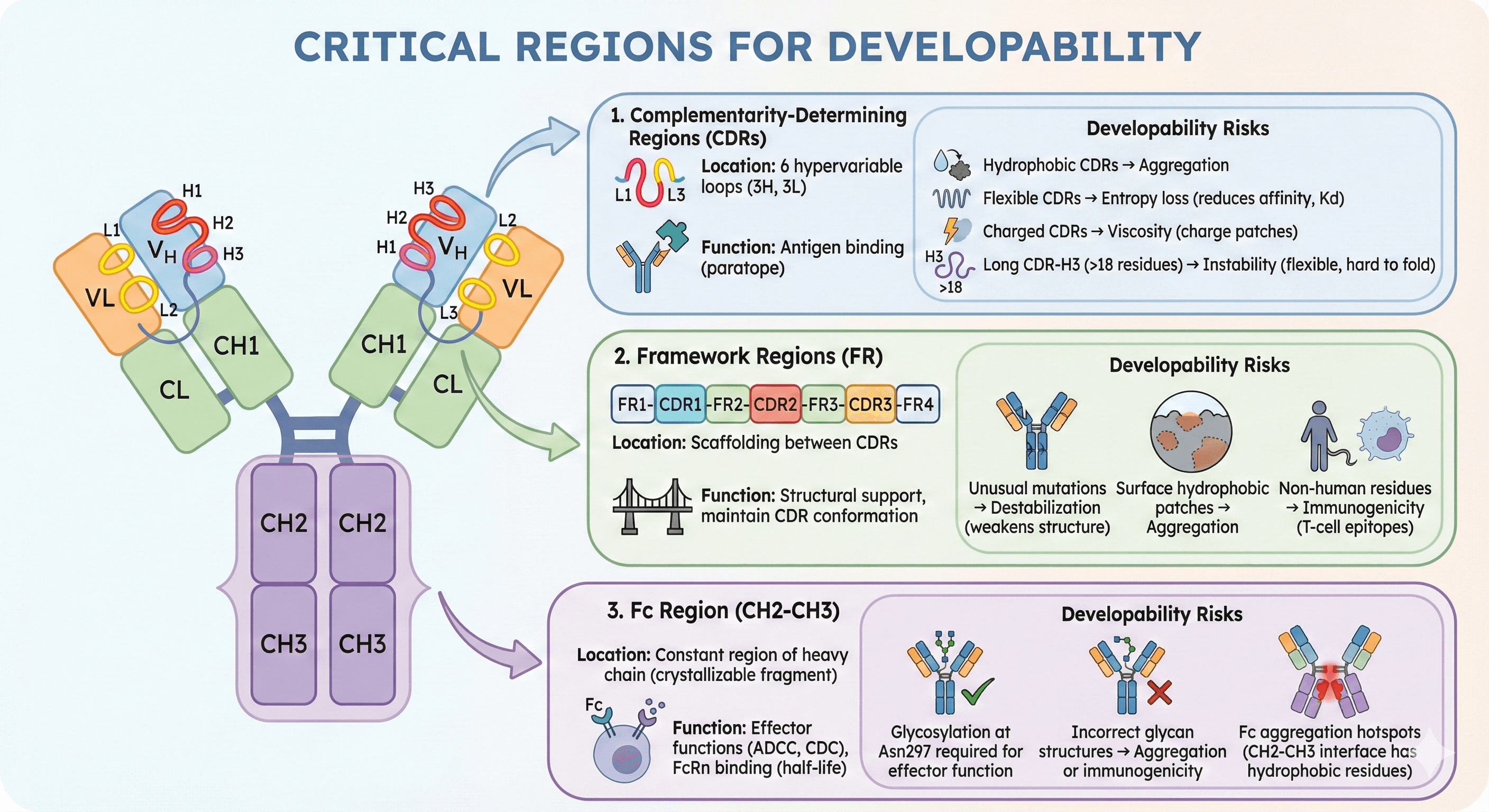
Critical Regions for Developability
1. Complementarity-Determining Regions (CDRs)
Location: 6 hypervariable loops (3 on heavy chain: H1, H2, H3; 3 on light chain: L1, L2, L3)
Function: Antigen binding (paratope)
Developability risks:
Hydrophobic CDRs → aggregation
Flexible CDRs → entropy loss (reduces binding affinity, Kd affected)
Charged CDRs → viscosity (charge patches)
Long CDR-H3 (>18 residues) → instability (flexible loop, hard to fold correctly)
2. Framework Regions (FR)
Location: Scaffolding between CDRs (FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4)
Function: Structural support, maintain CDR conformation
Developability risks:
Unusual mutations → destabilization (non-consensus residues weaken structure)
Surface hydrophobic patches → aggregation
Non-human residues → immunogenicity (T-cell epitopes)
3. Fc Region (CH2-CH3)
Location: Constant region of heavy chain (crystallizable fragment)
Function: Effector functions (ADCC, CDC), FcRn binding (half-life)
Developability risks:
Glycosylation at Asn297 required for effector function (ADCC/CDC needs glycan)
Incorrect glycan structures → aggregation or immunogenicity
Fc aggregation hotspots (CH2-CH3 interface has hydrophobic residues)
Real-World Failure Modes
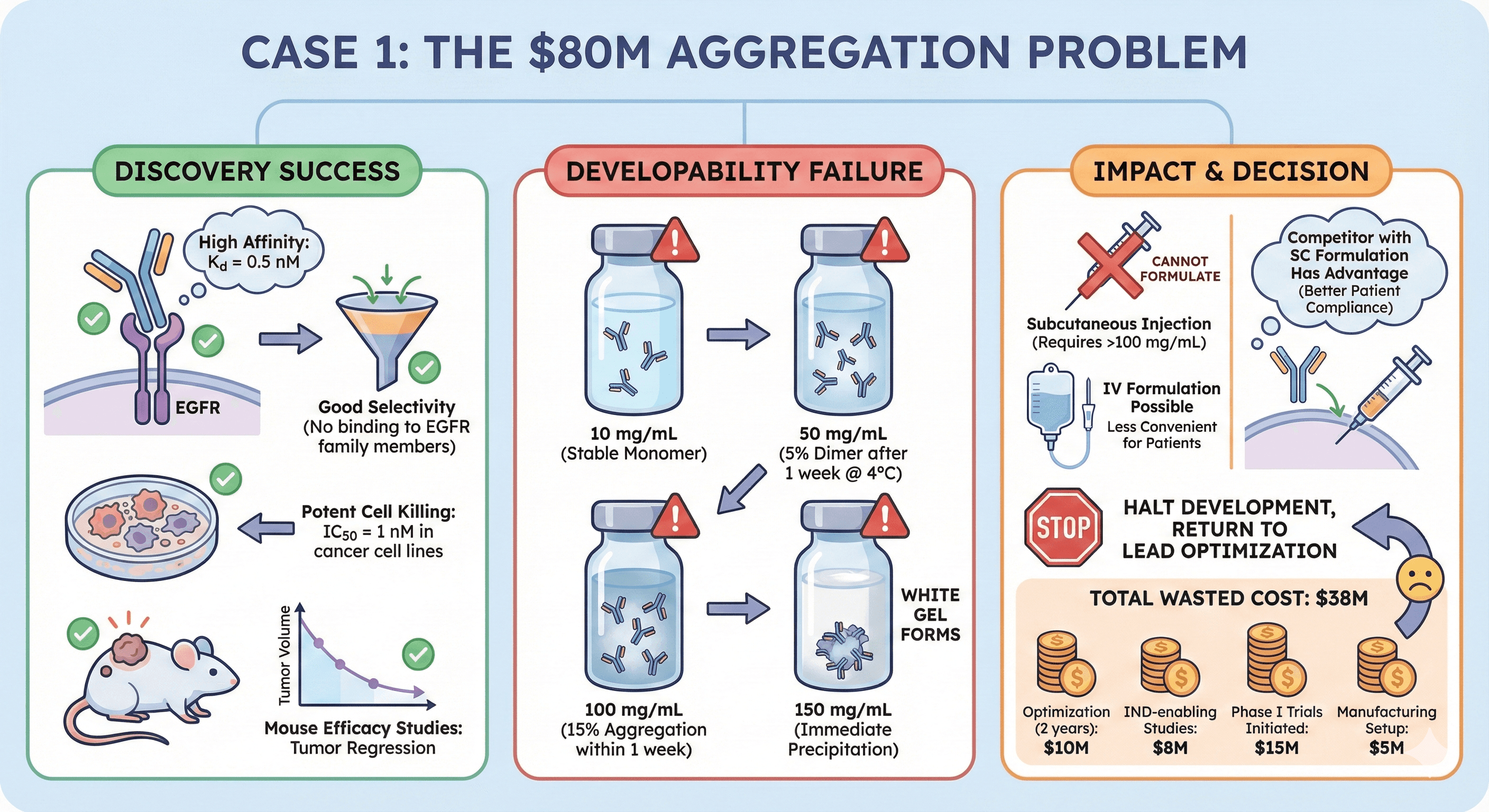
Case 1: The $80M Aggregation Problem
Candidate: Anti-EGFR antibody for solid tumors
Discovery success:
High affinity: Kd = 0.5 nM
Good selectivity (no binding to EGFR family members)
Potent cell killing: IC50 = 1 nM in cancer cell lines
Mouse efficacy studies: Tumor regression in xenograft models
Developability failure:
At 10 mg/mL: Stable (monomer)
At 50 mg/mL: 5% dimer after 1 week at 4°C
At 100 mg/mL: 15% aggregation within 1 week
At 150 mg/mL: Immediate precipitation (white gel forms)
Impact:
Cannot formulate for subcutaneous injection (requires >100 mg/mL)
IV formulation possible (lower concentration), but less convenient for patients
Competitor with SC formulation has advantage (better patient compliance)
Decision: Halt development, return to lead optimization
Cost:
2 years in lead optimization: $10M
IND-enabling studies: $8M
Phase I trials initiated: $15M
Manufacturing setup: $5M
Total wasted: $38M
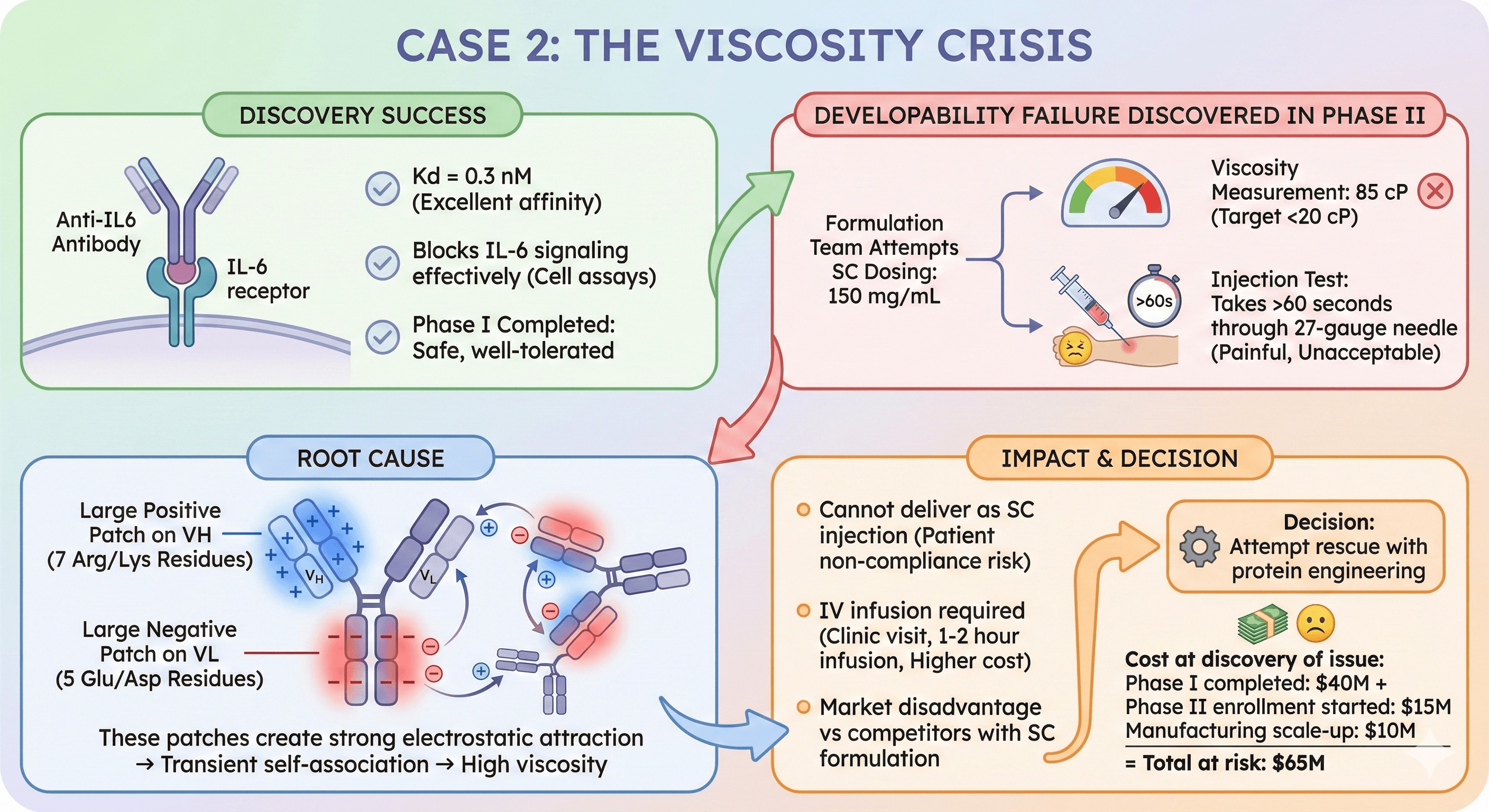
Case 2: The Viscosity Crisis
Candidate: Anti-IL6 antibody for rheumatoid arthritis
Discovery success:
Kd = 0.3 nM (excellent affinity)
Blocks IL-6 signaling effectively (cell assays)
Phase I completed: Safe, well-tolerated
Developability failure discovered in Phase II:
Formulation team attempts SC dosing: 150 mg/mL
Viscosity measurement: 85 cP (target <20 cP)
Injection test: Takes >60 seconds through 27-gauge needle (painful, unacceptable)
Root cause:
Charge patch analysis: Large positive patch on VH (7 Arg/Lys residues)
Large negative patch on VL (5 Glu/Asp residues)
These patches create strong electrostatic attraction → transient self-association → high viscosity
Impact:
Cannot deliver as SC injection (patient non-compliance risk)
IV infusion required (clinic visit, 1-2 hour infusion, higher cost)
Market disadvantage vs competitors with SC formulation
Decision: Attempt rescue with protein engineering
Cost at discovery of issue:
Phase I completed: $40M
Phase II enrollment started: $15M
Manufacturing scale-up: $10M
Total at risk: $65M
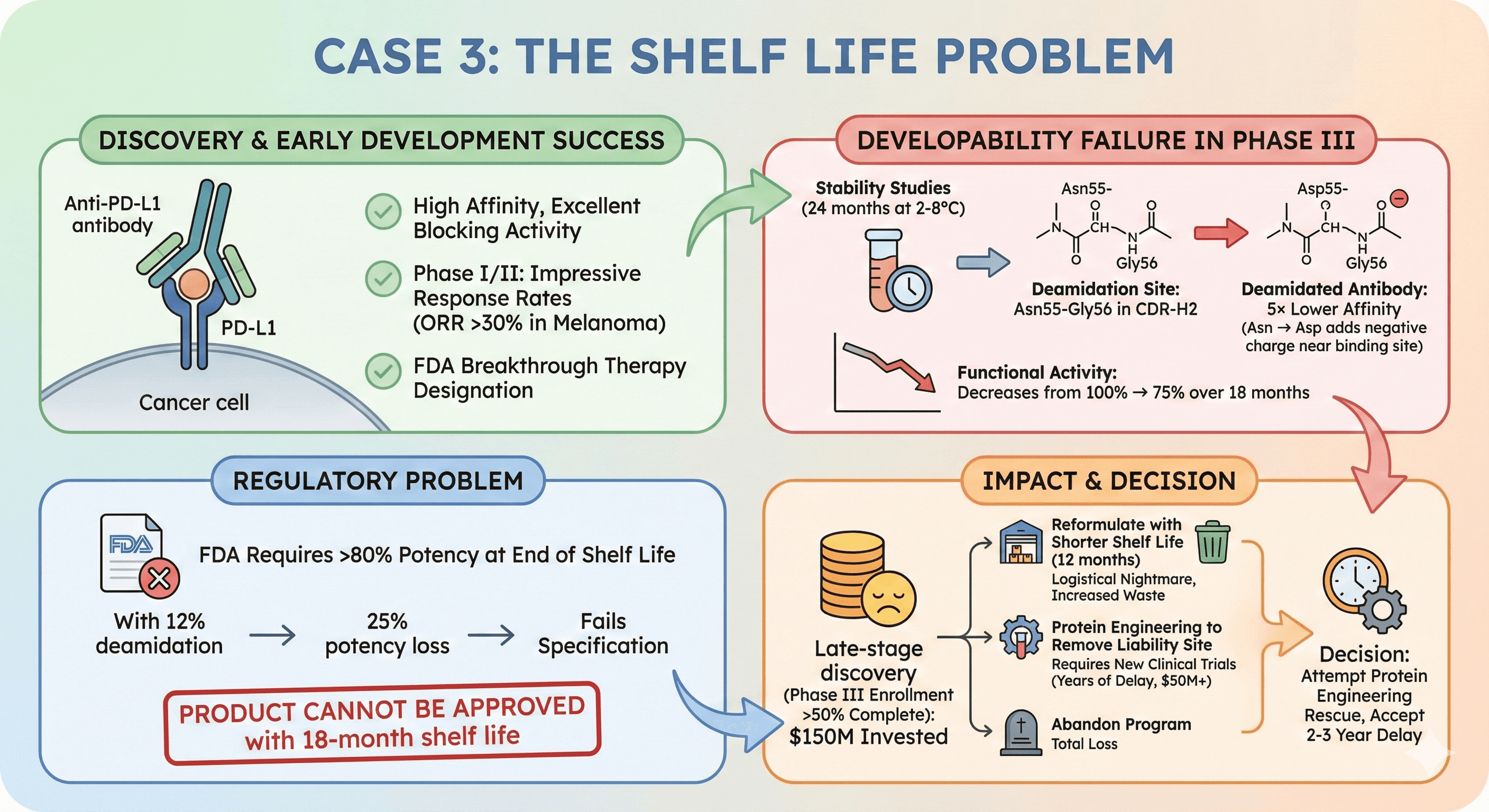
Case 3: The Shelf Life Problem
Candidate: Anti-PD-L1 antibody for cancer immunotherapy
Discovery & early development success:
High affinity, excellent blocking activity
Phase I/II: Impressive response rates (ORR >30% in melanoma)
FDA breakthrough therapy designation
Developability failure in Phase III:
Stability studies (24 months at 2-8°C): 12% deamidation
Deamidation site: Asn55-Gly56 in CDR-H2
Deamidated antibody: 5× lower affinity (Asn → Asp adds negative charge near binding site)
Functional activity: Decreases from 100% → 75% over 18 months
Regulatory problem:
FDA requires >80% potency at end of shelf life
With 12% deamidation → 25% potency loss → fails specification
Product cannot be approved with 18-month shelf life
Impact:
Late-stage discovery (Phase III enrollment >50% complete): $150M invested
Options:
Reformulate with shorter shelf life (12 months) → logistical nightmare, increased waste
Protein engineering to remove liability site → requires new clinical trials (years of delay, $50M+)
Abandon program → total loss
Decision: Attempt protein engineering rescue, accept 2-3 year delay
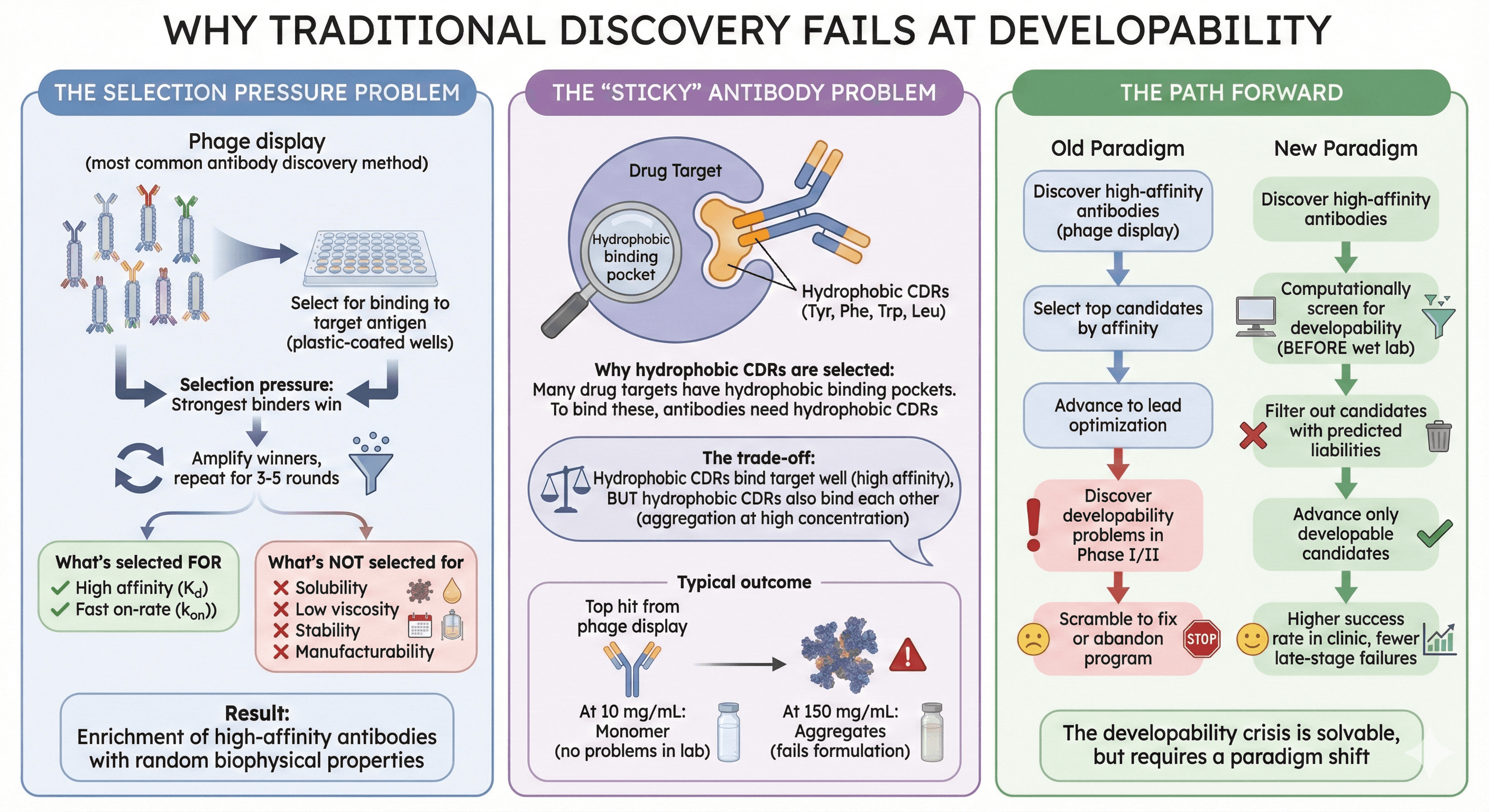
Why Traditional Discovery Fails at Developability
The Selection Pressure Problem
Phage display (most common antibody discovery method):
Display antibody fragments on phage surface
Select for binding to target antigen (plastic-coated wells)
Selection pressure: Strongest binders win
Amplify winners, repeat for 3-5 rounds
What's selected FOR:
High affinity (Kd)
Fast on-rate (kon)
What's NOT selected for:
Solubility (phage particles don't aggregate, so no selection pressure)
Low viscosity (not measured)
Stability (short-term selection, weeks not months)
Manufacturability (small-scale expression, not CHO bioreactor)
Result: Enrichment of high-affinity antibodies with random biophysical properties
The "Sticky" Antibody Problem
Why hydrophobic CDRs are selected:
Many drug targets have hydrophobic binding pockets (kinase ATP sites, GPCR ligand pockets)
To bind these pockets, antibodies need hydrophobic CDRs (Tyr, Phe, Trp, Leu)
Phage display strongly selects for these "sticky" CDRs (highest affinity)
The trade-off:
Hydrophobic CDRs bind target well (high affinity)
But hydrophobic CDRs also bind each other (aggregation at high concentration)
Typical outcome:
Top hit from phage display: Kd = 0.1 nM, CDR-H3 has 5 aromatic residues
At 10 mg/mL: Monomer (no problems in lab)
At 150 mg/mL: Aggregates (fails formulation)
The Path Forward
The developability crisis is solvable, but requires a paradigm shift:
Old paradigm:
Discover high-affinity antibodies (phage display)
Select top candidates by affinity
Advance to lead optimization
Discover developability problems in Phase I/II
Scramble to fix or abandon program
New paradigm:
Discover high-affinity antibodies
Computationally screen for developability (BEFORE wet lab)
Filter out candidates with predicted liabilities
Advance only developable candidates
Higher success rate in clinic, fewer late-stage failures
Key Takeaway
Developability is not optional. It's the difference between a $20B blockbuster drug and a $150M write-off.
The antibodies that make it to patients aren't just the ones with the highest affinity—they're the ones that can be manufactured at scale, stored for years, and injected painlessly.
Modern AI tools can predict these properties before you invest millions in clinical trials. The question is: Are you screening for developability early enough?
Ready to Assess Your Antibody's Developability?
If you have an antibody candidate and want to predict aggregation, viscosity, or stability issues before investing in expensive development, Orbion can help.
Orbion provides:
Aggregation hotspot prediction with mutation suggestions
Thermal stability optimization (ΔΔG predictions)
PTM liability detection (deamidation, oxidation, glycosylation)
Complete developability scorecard in minutes